SUMMARY AND EXPLANATION THE TEST
Typhoid fever is caused by S. typhi, a Gram-negative bacterium. World-wide an estimated 17 million cases and 600,000 associated deaths occur annually. Patients who are infected with HIV are at significantly increased risk of clinical infection with S. typhi. Evidence of H. pylori infection also presents an increase risk of acquiring typhoid fever. 1-5% of patients become chronic carrier harboring S. typhi in the gallbladder.
The clinical diagnosis of typhoid fever depends on the isolation of S. typhi from blood, bone marrow or a specific anatomic lesion. In the facilities that can not afford to perform this complicated and time-consuming procedure, Filix-Widal test is used to facilitate the diagnosis. However, many limitations lead to difficulties in the interpretation of the Widal test.
In contrast, theTyphoid IgG/IgM Rapid Test Kit is a simple and rapid laboratory test. The test simultaneously detects and differentiates the IgG and the IgM antibodies to S. typhi specific antigen in whole blood specimen thus aid in the determination of current or previous exposure to the S. typhi.
PRINCIPLE
The Typhoid IgG/IgM Combo Rapid Test is a lateral flow chromatographic
immunoassay. The test cassette consists of: 1) a burgundy colored conjugate pad containing recombinant S. typhoid H antigen and O antigen conjugated with colloid gold (Typhoid conjugates) and rabbit IgG-gold conjugates, 2) a nitrocellulose membrane strip containing two test bands (M and G bands) and a control band (C band). The M band is pre-coated with monoclonal anti-human IgM for the detection of IgM anti-S. typhi, G band is pre-coated with reagents for the detection of IgG
anti-S. typhi, and the C band is pre-coated with goat anti rabbit IgG.
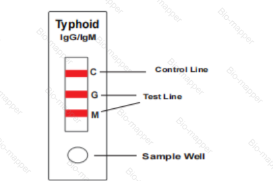
When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. Anti-S. typhi IgM if present in the specimen will bind to the Typhoid conjugates. The immunocomplex is then captured on the membrane by the pre-coated anti-human IgM antibody, forming a burgundy colored M band, indicating a S. typhi IgM positive test result.
Anti-S. typhi IgG if present in the specimen will bind to the Typhoid conjugates. The immunocomplex is then captured by the pre-coated reagents on the membrane, forming a burgundy colored G band, indicating a S. typhi IgG positive test result.
Absence of any test bands (M and G) suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti rabbit IgG/rabbit IgG-gold conjugate regardless of the color development on any of the test bands. Otherwise, the test result is invalid and the specimen must be retested with another device.